Abstract
Background: Chimeric antigen receptor T-cell (CAR T) therapy is a revolutionary cancer treatment that results in durable remission and/or cure for more than 50% of patients with relapsed/refractory hematologic malignancies without other viable options. Yet, a large proportion of patients do not respond to CAR T and/or will subsequently experience disease progression or relapse. Studies in cancer survivorship show that patient-reported outcomes (PROs) (i.e., health information reported directly by patients) can predict important cancer treatment outcomes, but associations have not been explored in the context of CAR T. To address this gap, we examined preliminary associations between PROs and response to CAR T therapy using graphical Bayesian networks analysis, an explainable machine learning approach that allows causal inferencing.
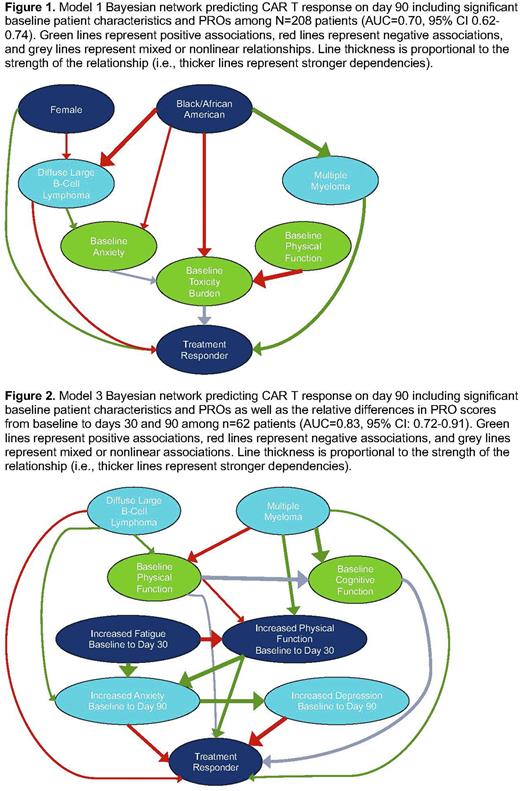
Methods: Data were pooled from four observational studies at Moffitt Cancer Center that collected common PROs among patients treated with CAR T. At baseline (i.e., pre-CAR T) and day 90 post-CAR T, patients completed measures from the PRO Measurement Information System (PROMIS) assessing anxiety, depression, fatigue, sleep disturbance, physical function, and pain interference. A subset of patients also completed PROMIS measures on day 30, including measures of global pain, cognitive function, and participation in social roles/activities. Higher scores indicated more of the construct measured. Patients also completed items from the PRO version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE) assessing patient-reported toxicities (e.g., decreased appetite, nausea, constipation, diarrhea) at baseline and days 30 and 90. PRO-CTCAE items were used to calculate a Toxicity Index measuring self-perceived toxicity burden. Higher scores indicated worse toxicity burden. Bayesian network analysis was used to examine three models for predicting CAR T treatment response on day 90, with patients categorized as responders (i.e., partial response or better) or non-responders (i.e., stable or progressive disease or death). Model 1 included baseline patient characteristics and PROs (i.e., anxiety, depression, fatigue, sleep disturbance, physical function, pain interference, toxicity burden). Model 2 added the relative difference in PRO scores between baseline and day 90. In a subset of patients, Model 3 added additional PROs (i.e., global pain, cognitive function, social function) and the relative difference in PRO scores between baseline and day 30. Toxicity burden was excluded from Model 3 due to missingness. The predictive performance of each model is reported as area under the receiver operating characteristics curve (AUC) with 95% confidence intervals (CI) based on 2000 stratified bootstrap replicates.
Results: Data from N=208 patients were analyzed. Most patients were male (58%), White (87%), and non-Hispanic (90%). Average age was 61 years old (range 19-82). Half were diagnosed with diffuse large B-cell lymphoma (52%), and 70% responded to CAR T by day 90. Model 1 yielded an AUC of 0.70 (95% CI: 0.62-0.74) and identified 6 constructs related to CAR T response: gender, race, cancer type, baseline anxiety, baseline physical function, and baseline toxicity burden (Figure 1). Model 2 was not significantly better than Model 1 (AUC=0.73, 95% CI: 0.67-0.80; Delong's test p-value=0.317) and identified 8 constructs related to CAR T response: gender, race, cancer type, baseline physical function, baseline pain interference, baseline toxicity burden, 90-day change in fatigue, and 90-day change in depression. Among a subset of n=62 patients, Model 3 improved the AUC to 0.83 (95% CI: 0.72-0.91) and was significantly better than Model 1 (Delong's test p-value=0.015) and Model 2 (Delong's test p-value=0.035). Model 3 identified 7 constructs related to CAR T response: cancer type, baseline physical function, baseline cognitive function, 30-day change in fatigue, 30-day change in physical function, 90-day change in anxiety, and 90-day change in depression (Figure 2).
Conclusions: Bayesian network models including baseline patient characteristics and longitudinal PROs were related to CAR T treatment response at day 90 with up to 83% predictive performance. These models are preliminary and should be validated in larger samples. This was a first step in identifying patient-reported predictors of CAR T clinical outcomes.
Disclosures
Gonzalez:Elly Health, Inc.: Membership on an entity's Board of Directors or advisory committees; SureMed Compliance: Consultancy. Hansen:BMS IMW Ide-Cel Academic Advisory Board: Membership on an entity's Board of Directors or advisory committees; Survivorship: Honoraria; OncLive: Honoraria. Jain:Novartis: Consultancy; BMS: Consultancy; MyeloidTx: Consultancy; Incyte: Research Funding; Kite Pharma: Consultancy, Research Funding. Locke:Society for Immunotherapy of Cancer: Other: Education or editorial role; Novartis: Membership on an entity's Board of Directors or advisory committees; Legend Biotech: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Leukemia and Lymphoma Society: Research Funding; National Cancer Institute: Research Funding; Umoja: Membership on an entity's Board of Directors or advisory committees; Kite Pharma: Research Funding; Allogene: Research Funding; Novartis: Research Funding; Cowen: Consultancy; BMS: Research Funding; ASH: Other: Education or editorial role; Emerging Therapy Solutions: Consultancy; Iovance: Membership on an entity's Board of Directors or advisory committees; BioPharma Communications CARE Education: Other: Education or editorial role; Aptitude Health: Other: Education or editorial role; Moffitt Cancer Center: Patents & Royalties: several patents held by the institution in his name (unlicensed) in the field of cellular immunotherapy; EcoR1: Consultancy; Clinical Care Options Oncology: Other: Education or editorial role; Gerson Lehrman Group: Consultancy; BlueBird Bio: Research Funding; Cellular Biomedicine Group: Membership on an entity's Board of Directors or advisory committees; GammaDelta Therapeutics: Membership on an entity's Board of Directors or advisory committees; Sana: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Wugen: Membership on an entity's Board of Directors or advisory committees; Caribou: Membership on an entity's Board of Directors or advisory committees; Calibr: Membership on an entity's Board of Directors or advisory committees; BMS/Celgene: Membership on an entity's Board of Directors or advisory committees; Bluebird Bio: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Allogene: Membership on an entity's Board of Directors or advisory committees; Kite Pharma: Membership on an entity's Board of Directors or advisory committees; Imedex: Other: Education or editorial role; A2: Membership on an entity's Board of Directors or advisory committees. Kirtane:Seattle Genetics: Current equity holder in private company; Oncternal Therapeutics: Current equity holder in private company; Veru: Current equity holder in private company. Jim:Merck: Consultancy; Janssen Scientific Affairs: Consultancy; Kite Pharma: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal